Cell Line Profile HL60
(ECACC catalogue no. 98070106)
Cell line history
HL60 is a human cell line derived from peripheral blood lymphocytes of a 36 year old woman suffering from acute promyleocytic leukaemia. It was one of the first human myeloid leukaemia cells to be established as a continuous suspension cell culture which had previously been difficult to achieve. Some attempts to create a long-term human myeloid leukaemia suspension using the Epstein-Barr virus (EBV) had occasionally generated cell lines, however it was found that these cells originated from other lymphocytes, not the leukaemia myeloid cells (Collins et al, 1977).
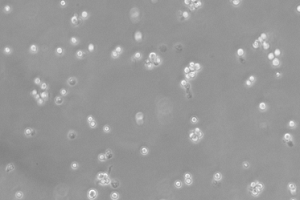

48 hours post resuscitation. Prior to cryopreservation.
Key characteristics
HL60 is a suspension cell line with a lymphoblastic morphology. It is known that around 10% of HL60 cells spontaneously differentiate (Gallagher et al, 1979); however differentiation can also be stimulated by a number of polar planar compounds such as DMF (0.8% v/v), and DMSO (1% - 1.5% v/v) and other known inducers of differentiation such as hypoxanthine, actinomycin D, retinoic acid (Fleck et al, 2005) or mixtures of retinoic acid and sodium butyrate (He and Breitman, 1991). HL60 cells can also be induced towards a monocyte/macrophage morphology using sodium butyrate alone (Martinez et al, 2002) or phorbol myristic acid, (PMA) (Padilla et al, 2000).
Applications
This cell line can be used as a model to study the cellular and molecular events involved in the proliferation and differentiation of leukaemic cells (Collins, 1987). It has also been used to produce extracellular vesicles (EVs) in studies aiming to understand the effects of leukaemic-derived EVs on human stem cells (Lavoie et al, 2018). HL60 cells, differentiated with 0.8% DMF for 4-5 days, are widely accepted as the model for differentiated phagocytic cells and granulocytes in opsonophagocytosis studies for a range of vaccine targets, including Group B Streptococcus (Guttormsen et al, 2008), Streptococcus pneumoniae (Romero-Steiner et al, 1997) and Neisseria meningitidis (Humphries et al, 2005). Finally, HL60 cells have been used in cancer research projects, for example a study into the role of MicroRNA-92a in development and progression of various cancers (Kohram et al, 2018) or to understand the synergistic role of chemotherapy agents in the treatment of leukaemia (Breitman and He, 1990).
Culture tips
HL60 cells should be cultured in RPMI 1640 + 2mM Glutamine + 10-20% Foetal Bovine Serum (FBS). At ECACC it has been found to be difficult to recover cells of acceptable viability after freezing in 10% glycerol/90% FBS. We recommend using 10% DMSO/90% FBS; however it must be removed from the culture by centrifugation immediately on thawing. This is because although DMSO gives a better recovery from frozen, it is a potent driver of differentiation so its removal is essential. The recommended tips for culturing HL60 are as follows: when starting from a frozen ampoule, add the thawed cells to a conical based centrifuge tube and add 4ml of culture medium. Take a sample of around 100ul to perform a cell count, and centrifuge the suspension at a low speed to prevent damage to the cells (i.e. 100 – 150 x g for 5 minutes). Remove the supernatant (containing the DMSO) and resuspend in fresh medium containing 20% FBS to help the cells attach and begin to proliferate. The cells should be left to incubate at 37°C; 5% CO2, and be checked daily. Maintain the cell culture between 1-9 x 100,000 cells/ml, and once the culture is established the FBS concentration can be reduced to 10%. After six weeks in culture, the cells may begin to differentiate.
|
Related cell lines |
ECACC catalogue number |
Description |
|---|---|---|
|
HL60 15-12 |
Human promyelocytic leukaemic cells isolated from HL60 cells capable of differentiating towards either neutrophils of monocytes with treatment of appropriate agents. |
|
|
HL60 Ast.3 |
Human promyelocytic leukaemic cells isolated from HL60 cells after treating cells with alpha-particle radiation and then cloned in medium containing 1.25% DMSO. Cells are capable of differentiating into neutrophils by induction with 1.75% DMSO. |
|
|
HL60 Ast.4 |
Human promyelocytic leukaemic cells isolated from HL60 cells after treating cells with alpha-particle radiation and then cloned in medium containing 1.25% DMSO. Treatment with 50nM TPA results in only very limited differentiation towards basophilic morphology (Giemsa stain). |
|
|
HL60 M2 |
Human promyelocytic leukaemic cells derived from HL60 cells induced to mature into neutrophils with 1.75% DMSO after treating with SV40. Fails to express SV40 specific T Ag suggesting that HL60 M2 represents expansion of sub-clones of HL60 with inherent restricted capacity for neutrophil differentiation. |
|
|
HL60 M4 |
Human promyelocytic leukaemic cells derived from HL60 cells induced to mature into neutrophils with 1.75% DMSO after treating with SV40. Fails to express SV40 specific T Ag suggesting that HL60 M4 represents expansion of sub-clones of HL60 with inherent restricted capacity for neutrophil differentiation. |
|
|
Eos-HL-60 |
Human promyelocytic leukaemic cells established from HL60 cells by sub-cloning in methylcellulose. Although the cells are capable of reverting to the parental phenotype, most passages maintain a high degree of eosinophil differentiation.Eos-HL-60 and HL-60 have been shown to be identical genetically by STR profiling |
|
|
Clone 15 HL-60 |
Human promyelocytic leukaemic cells derived from HL60 cells cultured at pH7.6-7.8 for 2 months. |
Key references
-
Collins, S.J., R.C. Gallo, and R.E. Gallagher, Continuous growth and differentiation of human myeloid leukaemic cells in suspension culture. Nature, 1977. 270: p. 347.
-
Gallagher, R., et al., Characterization of the continuous, differentiating myeloid cell line (HL-60) from a patient with acute promyelocytic leukemia. Blood, 1979. 54(3): p. 713-733.
-
Fleck, R.A., S. Romero-Steiner, and M.H. Nahm, Use of HL-60 Cell Line To Measure Opsonic Capacity of Pneumococcal Antibodies. Clinical and Diagnostic Laboratory Immunology, 2005. 12(1): p. 19-27.
-
He, R.Y. and T.R. Breitman, Retinoic acid inhibits sodium butyrate-induced monocytic differentiation of HL60 cells while synergistically inducing granulocytoid differentiation. Eur J Haematol, 1991. 46(2): p. 93-100.
-
Martinez, J., et al., Opsonophagocytosis of fluorescent polystyrene beads coupled to Neisseria meningitidis serogroup A, C, Y, or W135 polysaccharide correlates with serum bactericidal activity. Clin Diagn Lab Immunol, 2002. 9(2): p. 485-8.
-
Padilla, P.I., et al., Morphologic differentiation of HL-60 cells is associated with appearance of RPTPbeta and induction of Helicobacter pylori VacA sensitivity. J Biol Chem, 2000. 275(20): p. 15200-6.
-
Collins, S., The HL-60 promyelocytic leukemia cell line: proliferation, differentiation, and cellular oncogene expression. Blood, 1987. 70(5): p. 1233-1244.
-
Lavoie, J.R., et al., 52 - Characterization of leukemia-derived extracellular vesicles and subsequent effects on mesenchymal stem cell phenotype. Cytotherapy, 2018. 20(5, Supplement): p. S22.
-
Guttormsen, H.K., Y. Liu, and L.C. Paoletti, Functional activity of antisera to group B streptococcal conjugate vaccines measured with an opsonophagocytosis assay and HL-60 effector cells. Hum Vaccin, 2008. 4(5): p. 370-4.
-
Romero-Steiner, S., et al., Standardization of an opsonophagocytic assay for the measurement of functional antibody activity against Streptococcus pneumoniae using differentiated HL-60 cells. Clin Diagn Lab Immunol, 1997. 4(4): p. 415-22.
-
Humphries, H.E., et al., Seroprevalence of Antibody-Mediated, Complement-Dependent Opsonophagocytic Activity against Neisseria meningitidis Serogroup B in England. Clinical and Vaccine Immunology : CVI, 2015. 22(5): p. 503-509.
-
Fatemeh, K., et al., Cell type‐dependent functions of microRNA‐92a. Journal of Cellular Biochemistry, 2018. 0(0).
-
Breitman, T.R. and R.Y. He, Combinations of retinoic acid with either sodium butyrate, dimethyl sulfoxide, or hexamethylene bisacetamide synergistically induce differentiation of the human myeloid leukemia cell line HL60. Cancer Res, 1990. 50(19): p. 6268-73.
