Cell Line Profile CACO-2
(ECACC catalogue no. 09042001)
Cell Line History
CACO-2 was first isolated in 1977 from a 72-year-old Caucasian male using the explant culture technique [1]. Although isolated from a colonic tumour, upon differentiation, the CACO2 cell line demonstrated morphological and biochemical characteristics of small intestine enterocytes [2, 3].
Key characteristics
In culture, CACO-2 cells grow as a cylindrical polarised monolayer, where brush border enzyme secreting microvilli develop on the apical side, and uniform tight junctions form between adjacent cells [4]. After reaching confluence, domes will form in the cultures, due to the unidirectional flux of ions and water through the polarised monolayer. CACO-2 is a heterogenous culture, which is characterised by subpopulations of differing morphologies [5].
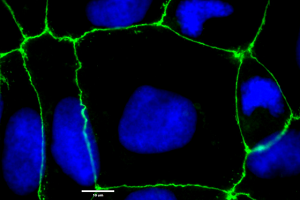
Figure 1: CACO-2 cells showing uniform tight junction formation. Cells were cultured on to coverslips in 6 well plates and allowed to differentiate for approx. 17 days. Cells were stained for ZO-1 protein using a rabbit IgG polyclonal primary antibody, an Alexafluor conjugated goat anti-rabbit secondary antibody (green) and NucBlue™ (blue) counterstaining. Images were taken at magnification x100.
Applications
CACO-2 is a key cell line in the drug discovery workflow, providing the principle in vitro intestinal epithelium cellular model for drug absorption and permeability [6]. Additionally, CACO-2 is commonly utilised to study drug transport mechanisms, due to the presence of many functional brush border enzymes and transport proteins [7]. CACO-2 cultures can also be applied to study epithelial tight junction barrier formation [8] (Figure 1 & 2).
STR Profile
|
Locus |
Score |
|---|---|
|
Amelogenin |
X,X |
|
vWA |
16,18 |
|
CSF1PO |
11 |
|
D13S317 |
11,13,14 |
|
D16S539 |
12,13 |
|
D18S51 |
12 |
|
D21S11 |
30 |
|
D3S1358 |
14,17 |
|
D5S818 |
12,13 |
|
D7S820 |
11,12 |
|
D8S1179 |
12,14 |
|
FGA |
19 |
|
Penta D |
9,11 |
|
Penta E |
7 |
|
THO1 |
6 |
|
TPOX |
9,11 |
Culture Tips
Split sub-confluent cultures when at 70-80% confluence, and seed at 2-4x10,000 cells/cm² using 0.25% trypsin or trypsin/EDTA.
Cells should be cultured in EMEM (EBSS) + 2mM Glutamine + 1% Non-Essential Amino Acids (NEAA) + 10% Foetal Bovine Serum (FBS) at 5% CO2; 37°C.
CACO-2 cells will reach full confluence after approximately 4 days in culture when seeded at 2-4x10,000 cells/cm².
CACO-2 cells will require 17-21 days in culture in order to differentiate and demonstrate the desired characteristics of small intestine enterocytes [9].
Due to dome formation, cells may show the appearance of circular vacuoles in the cytoplasm. These can increase in frequency as the culture density increases to confluence. To reduce their frequency, media change confluent cultures after 2-3 days if not sub cultured.
CACO-2 tight junction expression is generally assessed by trans-epithelial electrical resistance (TEER).
For drug discovery assays, cells can be cultured on to semi-permeable supports (filter wells or “Transwells™”).
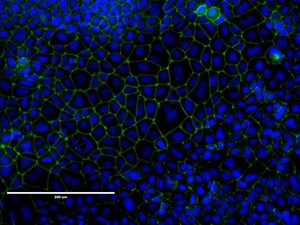
Figure 2: CACO-2 cells showing uniform tight junction formation. Heterogenous cell populations can be observed, which is typical of CACO-2 cultures. Cells were stained for ZO-1 protein using a rabbit IgG polyclonal primary antibody, an Alexafluor conjugated goat anti-rabbit secondary antibody.
|
Related cell lines |
ECACC Catalogue Number |
Description |
|---|---|---|
|
CACO-2 |
Human colon adenocarcinoma. This line has not undergone characterisation for intestinal permeability: Dome, microvilli and tight junction formation and transport functionality. |
|
|
HCT-8 |
Human ileocecal adenocarcinoma. |
|
|
IEC 6 |
Rat small intestine epithelia. |
|
|
HT29 |
Human Caucasian colon adenocarcinoma. |
|
|
HT29-MTXE12 |
Mucous-secreting HT29-MTX subclones were isolated from this cell clone and characterized. |
References
-
Fogh, J., J.M. Fogh, and T.J.J.o.t.N.C.I. Orfeo, One hundred and twenty-seven cultured human tumor cell lines producing tumors in nude mice. Journal of the National Cancer Institute. 1977. 59(1): p. 221-226.
-
Hidalgo, I., T. Raub, and R. Borchardt, Characterization of the human colon carcinoma cell line (Caco-2) as a model system for intestinal epithelial permeability. 1989. 96(2): p. 736-749.
-
Hilgers, A., R. Conradi, and P. Burton, Caco-2 cell monolayers as a model for drug transport across the intestinal mucosa. Pharmaceutical Research, 1990. 7(9): p. 902-910.
-
Sambuy, Y., et al., The Caco-2 cell line as a model of the intestinal barrier: influence of cell and culture-related factors on Caco-2 cell functional characteristics. Cell Biology and Toxicology. 2005. 21(1): p. 1-26.
-
Walter, E. and T. Kissel, Heterogeneity in the human intestinal cell line Caco-2 leads to differences in transepithelial transport. European Journal of Pharmaceutical Sciences, 1995. 3(4): p. 215-230.
-
Hubatsch, I., E.G. Ragnarsson, and P. Artursson, Determination of drug permeability and prediction of drug absorption in Caco-2 monolayers. Nature Protocols, 2007. 2(9): p. 2111.
-
Rubas, W., et al., Flux measurements across Caco-2 monolayers may predict transport in human large intestinal tissue. Journal of Pharmaceutical Sciences, 1996. 85(2): p. 165-169.
-
Lu, S., et al., Transport properties are not altered across Caco-2 cells with heightened TEER despite underlying physiological and ultrastructural changes. Journal of Pharmaceutical Sciences, 1996. 85(3): p. 270-273.
-
Natoli, M., et al., Good Caco-2 cell culture practices. Toxicology in Vitro, 2012. 26(8): p. 12431246.
