Cell Line Profile OE19
(ECACC catalogue no. 96071721)
Cell line history
OE19, also known as JROECL19, was originally one of five permanent human oesophageal adenocarcinoma (EAC) cell lines established in 1993. It is from a stage III tumour of a 72 year old male patient1 and deposited in ECACC by Drs J C Rockett/A Morris, Department of Biological Sciences, University of Warwick and Dr S J Darnton, Birmingham Heartlands Hospital. Boonstra et al. discovered that cell lines SEG-1, BIC-1, and SK-GT-5 are not EAC cell lines but large cell lung cancer cell line H460, colorectal adenocarcinoma cell line SW620, and gastric fundus carcinoma cell line SK-GT-2, respectively. However, they confirmed that cell lines FLO-1, KYAE-1, SK-GT-4, OE19, OE33, OACP4C, OACM5.1, ESO26, and ESO51 are derived from human EACs2. All nine of these verified EAC cell lines, together with their genotyping information, have been deposited with ECACC.
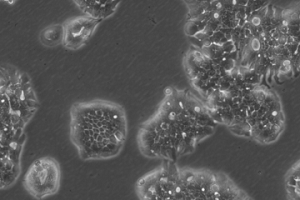
OE19 cells don’t tend to form a monolayer but instead grow in densely packed islands. The image was captured 8 days after resuscitation showing typical growth in islands.
Key characteristics
The cell line OE19 has weak expression of HLA-A, -B and -C antigens (MHC class I) and treatment with interferon-gamma induces the expression of ICAM-1 (CD54). Expression of HLA-DR (MHC class II) on interferon-gamma addition was only measured in a sub-population of OE19. The cells also express epithelial cytokeratins, are sensitive to the inhibitory effects of TGF-β1 on proliferation and are tumourigenic in nude mice1.
Applications
Primary applications of this cell line are in carcinogenesis and chemotherapeutic studies specifically for oesophageal adenocarcinomas3-8. These cells are known to be sensitive to the breast cancer drug lapatinib but can be cloned to achieve lapatinib resistance6. A combination of oleic acid and alpha linoleic acid down regulate proliferation of malignant OE19 cells – a finding that could be used in the chemoprevention or management of oesophageal cancers7. Interestingly, these cells have recently been found to express sensitivity to increasing concentrations of the antidiabetic drug Metformin which has been associated with a reduced cancer incidence5. OE19 cells also show clear nuclear translocation of p-STAT5 upon EGFR stimulation (promotes cellular proliferation)3 and the down-regulation the protein Survivin (BIRC5) in OE19 cells results in increased apoptosis8 which can be used as a tool in targeted therapies.
Culture tips
OE19 cells should be cultured using standard subculture methods in RPMI 1640 + 2mM Glutamine + 10% Foetal Bovine Serum (FBS). These cells are slow growers and may take up to 7 days to reach 70% confluency post resuscitation. We recommend a split ratio of 1:8 i.e. seeding density of 2-4 x104 cells/cm². Once past the first subculture these cells take 4-5 days to reach approximately 60% confluency when set up at 2 x104 cells/cm².
|
Related cell lines |
ECACC Catalogue number |
Description |
|---|---|---|
|
ESO26 |
Adenocarcinoma of the gastroesophageal junction |
|
|
ESO51 |
Distal oesophageal adenocarcinoma |
|
|
FLO-1 |
Distal oesophageal adenocarcinoma |
|
|
KYAE-1 |
Distal oesophageal adenocarcinoma |
|
|
KYSE-270 |
Human oesophageal squamous cell carcinoma |
|
|
KYSE-30 |
Human Asian squamous cell carcinoma |
|
|
KYSE-410 |
Human oesophageal squamous cell carcinoma |
|
|
KYSE-70 |
Human oesophageal squamous cell carcinoma |
|
|
OACM5.1 C |
Barretts adenocarcinoma, adenocarcinoma of distal oesophagus |
|
|
OE21 |
Human Caucasian oesophageal squamous cell carcinoma |
|
|
OE33 |
Human Caucasian oesophageal carcinoma |
|
|
SK-GT-2 |
Adenocarcinoma of the Gastric fundus, poorly differentiated |
|
|
SK-GT-4 |
Oesophagus adenocarcinoma, well-differentiated |
|
|
OACP4 C |
Gastric cardia adenocarcinoma |
Key references
-
Rockett, J.C., Larkin, K., Darnton, S.J., Morris, A.G. & Matthews, H.R. Five newly established oesophageal carcinoma cell lines: phenotypic and immunological characterization. British Journal of Cancer. 75(2): 258-263 (1997)
-
Boonstra, J.J., van Marion, R., Beer, D.G., Lin, L., Chaves, P., Ribeiro, C., et al. Verification and unmasking of widely used esophageal adenocarcinoma cell lines. JNCI Journal of the National Cancer Institute. 102(4): 271-274 (2010)
-
Spohn, L., Fichter, C., Werner, M. & Lassman, S. Subcellular localization of EGFR in esophageal carcinoma cell lines. J. Cell Commun. Signal. 10: 41-47 (2016)
-
Kresty, L.A., Weh, K.M., Zeysus-Johns, B., Perez, L.N., & Howell, A.B. Cranberry proanthocyanidins inhibit esophageal adenocarcinoma in vitro and in vivo through pleiotropic cell death induction and PI3K/AKT/mTOR inactivation. Oncotarget. 6(32): 33438-33455 (2015)
-
Fujihara, S., Kato, K., Morishita, A., Iwama, H., Nishioka, T., et al. Antidiabetic drug metformin inhibits esophageal adenocarcinoma cell proliferation in vitro and in vivo. International Journal of Oncology. 46: 2172-2180 (2015)
-
Hong, Y.S., Kim, J., Pectasides, E., Fox, C., Hong, S-W., et al. Src Mutation Induces Acquired Lapatinib Resistance in ERBB2-Amplified Human Gastroesophageal Adenocarcinoma Models. PLOS ONE. 9(10): e109440 (2014)
-
Moon, H-S., Batirel, S. & Mantzoros, C.S. Alpha linolenic acid and oleic acid additively down-regulate malignant potential and positively cross-regulate AMPK/S6 axis in OE19 and OE33 esophageal cancer cells. Metabolism Clinical and Experimental. 63: 1447-1454 (2014)
-
Malhotra, U., Zaidi, A.H., Kosovec, J.E., Kasi, P.M., Komatsu, Y., et al. Prognostic Balue and Targeted Inhibition of Survivin Expression in Esophageal Adenocarcinoma and Cancer-Adjacent Squamous Epithelium. PLOS ONE. 8(11): e78343 (2013)
