Cell Line Profile B95-8
(ECACC catalogue no. 85011419)
Cell line history
The B95-8 cell line’s name originates from the B95 strain of Epstein Barr Virus (EBV). The viral strain was isolated from a case of transfusion induced mononucleosis and subsequently used in vitro to immortalise monkey blood leukocytes (Miller et al, PNAS, 1972) to generate the cell line.
Through the use of DNA profiling, it has now been shown that the B95-8 cell line was most likely originally derived from the Cotton-top Tamarin Monkey Peripheral Blood Lymphocyte as opposed to the commonly assumed origin of a marmoset. All 172,282 base pairs of B95-8’s nucleotide sequence have been successfully mapped using the dideoxynucleotide/M13 sequencing procedure (Baer et al, Nature, 1984). This procedure uses DNA polymerase to synthesise DNA from a single stranded template, halting when one of four dideoxynucleotides (for each base) is incorporated. This incorporation produces an output which can be read, and after many cycles, gives the complete sequence of DNA (Yu, DNA Sequencing Methods, 2001). B95-8 is known for producing large quantities of EBV, however studies have shown that the DNA characterising this production was not present in the original strain. This therefore means that a mutation must have taken place shortly after the creation of the B95-8 cell line causing the production of EBV (Skare et al, JoV, 1982). EBV is a common human virus which spreads through bodily fluid (in particular saliva) and causes infections such as Mononucleosis (Mono) (Sumaya & Ench, Pediatrics, 2003).
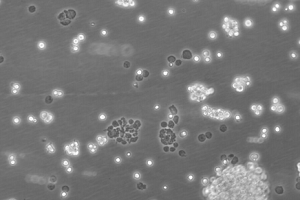
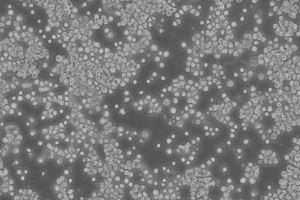
48 hours post resuscitation Prior to cryopreservation
Key characteristics
B95-8 cells originate from the blood of a cotton-top tamarin. The morphology of the cell line is predominantly a suspension of singular, ovular cells or clusters of small clumps, however roughly 10% of the cells remain adherent. The cells are lymphoblasts – immature cells which will become lymphocytes after antigenetic stimulation (Miller-Keane, 2003). Under normal cell culture conditions (37°C, 10% Serum) the cell line proliferates and does not produce optimum virus titres, however, reducing the temperature to 32°C and lowering the serum concentration results in highly efficient pathogenic virus production (Bolton & Spurr, 1996). B95-8 cells have also been reported to produce high titres of EBV when exposed to other EBV-inducing agents (Shaw et al, JoV, 1987)
Applications
The B95-8 cells are used as a source of EBV for the transformation and immortalisation of human B lymphoctyes into continuous cell lines (Romano et al, Nucleic Acids Research, 2009) (Shope et al, PNAS, 1973) (Bolton & Spurr, 1996). Being able to propagate cells indefinitely from specific donors ensures an indefinite supply of genomic material without the need to resample from the original donor. This technique has been used at ECACC to generate tens of thousands of patient specific lymphoblastoid cell lines over the last 30 years and was fundamental to the success of the UK human genome mapping project. An example of their use was in a genomewide scan for autism involving a resource of over 2000 cell lines generated by ECACC (Palferman et al. 2001). Cell lines immortalised by EBV transformation have been used to generate all the cell lines in the ECACC HLA Typed Collection and the Human Random Control DNA Panels.
Culture tips
B95-8 should be cultured in RPMI 1640 + 2mM Glutamine + 10% Foetal Bovine Serum (FBS).The culture should be maintained between 3-9 x 105 cells/ml at 37°C + 5% CO2. Due to its partial adherence, trypsin may be required to fully remove all of the cells from the flasks. During cryopreservation, the quantity of cells should be kept at a minimum of 8 x 106 cells/ampoule to ensure enough cells survive to resuscitate the cell line. These cells should be handled under laboratory containment level 2.
|
Related cell lines |
ECACC catalogue number |
Description |
|---|---|---|
|
B95a |
Derivative of B95-8 adapted for more adherent growth. Much more vulnerable to the measles virus so useful in the isolation of measles. Culture in DMEM +2mM Glutamine + 5% FBS. |
Key References
1. Miller G. Shope T. Lisco H. Stitt D. Lipman M. Epstein-Barr virus: transformation, cytopathic changes, and viral antigens in squirrel monkey and marmoset leukocytes. Proc. Natl. Acad. Sci. USA 69: 383-387, 1972. PubMed: 4333982
2. Baer, R., Bankier A. T., Biggin, M. D., et al. DNA sequence and expression of the B95-8 Epstein-Barr virus genome. Nature. 310(5974): pp207-11 (1984 Jul 19-24)
3. Yu, Q. M13 Sequencing. DNA Sequencing Protocols. Methods in Molecular Biology. Vol. 167pp33-38 (2001)
4. Skare, J., Edson, C., Farley, J., &Strominger, J. L. The B95-8 isolate of Epstein-Barr virusarose from an isolate with a standard genome. Journal of Virology. 44(3), pp1088-1091 (1982)
5. Sumaya, V. S., Ench, Y. Epstein-Barr Virus Infectious Mononucleosis in Children. Pediatrics. 75(6) (1985)
6. Lymphoblast. Miller-Keane Encyclopaedia and Dictionary of Medicine, Nursing, and Allied Health. Ver. 7 (2003)
7. Bolton, BJ. Spurr, NK. B-Lymphocytes. In Freshney, RI and Freshney, MG (ed): Culture of Immortalized Cells; Wiley-Liss, New York, 1996, pp 283-297
8. Shaw, J. E., Petit, R. G., Leung, K. Growth of B95-8 cells and expression of Epstein-Barr virus lytic phase in serum-free medium. Journal of Virology 61(12): pp4033-4037 (1987)
9. Romano, P. Manniello, A. Aresu, O. Armento, M. Cesaro, M. Parodi, O Cell Line Data Base: structure and recent improvements towards molecular authentication of human cell lines. Nucleic Acids Research. 37(Database issue):D925-D932. (2009)
10. Shope, T., Dechairo, D., Miller, G. Malignant Lymphoma in Cottontop Marmosets after Inoculation with Epstein-Barr virus. Proc. Nat. Acad. Sci. USA. 70(9) pp2487-2491 (1973)
11. Palferman, S. et al. A genomewide screen for autism: Strong evidence for linkage to chromosomes 2q, 7q, and 16p The American Journal of Human Genetics 69:570-581 (2001)
