A case of best practice
Indirect Hoechst stain using an indicator cell line
The most common causes of failure in a Tissue Culture lab are death of cultures, microbial or viral contamination and cross-contamination or misidentification.
One of the microbial contamination problems is that of Mycoplasma, micro-organisms belonging to the class Mollicutes (this includes the eponymous Mycoplasma family as well as other relatives such as Achiolaplasma). They are the smallest, free living, self-replicating organisms and were first thought to be viruses, however they can grow in cell-free media and contain both RNA and DNA and are therefore classified as bacteria.
Effects on the host cells are significant but not obvious, as despite being invisible to the naked eye they grow to a high density (they can out-number cells 100-1000-fold). They have little or no capacity to generate amino acids, fatty acids, vitamins, and co-factors so they are dependent on their hosts and immediate environment. They can affect the rate of growth and induce morphological changes of the cell line.
Mycoplasmas invalidate research. Testing is a requirement by regulatory bodies (FDA and EMEA) and particularly in cell lines used for diagnostics or production of therapeutic products.
Culture Collections Quality Control Team use several assays to detect mycoplasma in samples but the focus here is on Indirect Hoechst stain using an indicator cell line.
The dye used is Hoechst 33258 which was originally developed by the company Hoechst AG and numbered according to the of compounds developed. The indicator cell line we use is Vero (Vero ECACC 84113001) due to the increased susceptibility of the cells to mycoplasma infection.
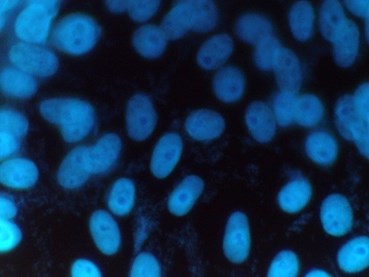
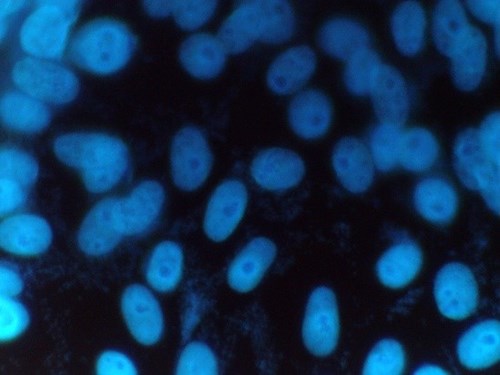
Figure 1: Mycoplasma positive sample
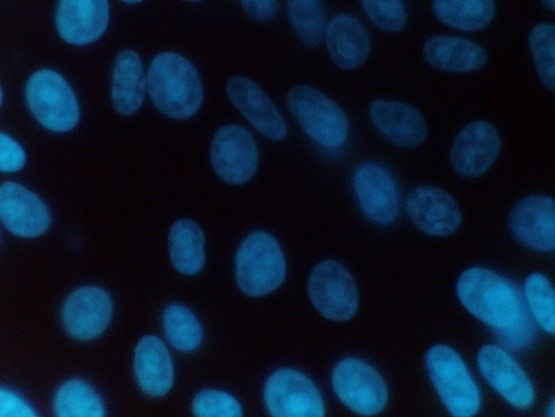
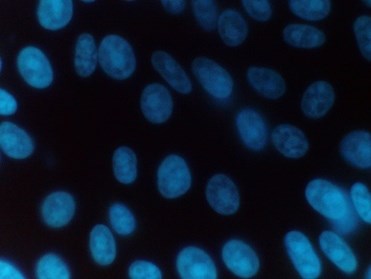
Figure 2: Mycoplasma negative sample
The process we use is as follows:
1. Once received, the test sample is aseptically inoculated onto Vero cells which have been grown on coverslips in 12 well tissue culture plates.
2. A negative and two positive control samples (Mycoplasma hyorhinis NCTC 10112 and Mycoplasma orale NCTC 10130) are also set up on a separate 12 well plate containing the indicator cell line.
3. All the samples are then incubated for 3-5 days after which the samples are fixed to the coverslip and stained using the fluorescent dye Hoechst 33258.
4. The fixed samples are then examined under UV epi fluorescence at x1000 magnification.
Positive samples are identified by their characteristic particulate or filamentous pattern of fluorescence on the cell surface or if contamination is heavy in the surrounding area (see figure 1.). Negative samples should demonstrate no evidence of fluorescing DNA outside of the cell membrane against the fluorescing Vero cell nuclei (see Figure 2.).
Written by Deborah Lister
